PATIENT GUIDANCE
LEAFLET
TRAXAM FOAM
(felbinac)
This leaflet provides brief information about your medicine. Please read it carefully before you start to use TRAXAM. For further information consult your doctor or pharmacist.
THE NAME OF YOUR PRODUCT
IS TRAXAM
TRAXAM foam is a white foam containing the analgesic and anti-inflammatory drug called felbinac. When rubbed into the affected area(s) the foam breaks down into a clear liquid which penetrates the skin and helps reduce pain and swelling.
THINGS TO REMEMBER ABOUT TRAXAM
- Make sure it is safe for you to use TRAXAM before you start (see page 3 of this leaflet).
- Use TRAXAM exactly as directed by your doctor. Read the pharmacist’s label carefully.
- TRAXAM is for External Use Only
- TRAXAM Foam is contained in a pressurised aerosol can. Use aerosols carefully - (see page 3 of this leaflet).
- Keep your medicine in a safe place out of the reach of children.
TELL YOUR DOCTOR BEFORE STARTING TRAXAM IF..
- You are pregnant or breast feeding, or
- aspirin or other similar medicines have given you asthma, itchy-rash or hayfever-like symptoms.
HOW TO USE TRAXAM
Using the foam
- Always hold the can upright.
- Press the nozzle and dispense foam onto the palm of your hand.
- Rub the foam lightly into the affected area(s). The foam changes to a clear liquid on contact with the skin.
- Be careful not to spray the can near your face or eyes. If any foam comes into contact with your eyes, rinse with cold water and consult your doctor if necessary.
- Do not apply TRAXAM on broken skin such as cuts and grazes or under dressings such as sticking plasters.
HOW MUCH AND HOW OFTEN?
- TRAXAM should be used as directed by your doctor. Read the Pharmacist’s label carefully.
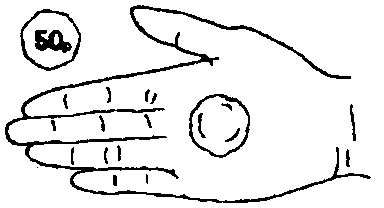
- Gently rub one golf ball sized (one and a half inches (4cm) in diameter.
See illustration) quantity of foam into the affected area(s).
TRAXAM is usually applied 2-4 times a day.
AFTER USING YOUR MEDICINE
- Like all medicines TRAXAM may cause side effects in a few people.
- You may experience redness of the skin or itching after applying TRAXAM. These symptoms disappear if you stop treatment.
- If you experience these or any other troublesome effects while using TRAXAM consult your doctor or pharmacist.
STORING YOUR MEDICINE
Store at room temperature (15 - 25°C).
- Keep all medicines in a safe place out of the reach of children.
- If your doctor decides to stop treatment return any remaining TRAXAM to your pharmacist or dispose of it safely.
- Do not use TRAXAM after the expiry date.
- FLAMMABLE - Please see can for warnings.
CONTENTS OF YOUR MEDICINE
- Active ingredient: felbinac (3.17% w/w).
- The foam contains other ingredients to make it easy to apply and give it a smooth feel.
- Contains no propellant alleged to damage ozone.
OTHER INFORMATION
TRAXAM Foam may not be suitable for use in young children.
REMEMBER: This medicine is for you. Only a doctor can prescribe it for you . Do not give it to anyone else even if their symptoms are the same as yours.
TRAXAM is a product of: LEDERLE LABORATORIES, Gosport, Hampshire, England.
Product Licence/Authorisation Numbers:
PL 0095/00238 PA 37/60/2
(Cyanamid of Great Britain Limited)
Distributed in Eire by:
T.P. Whelehan Son & Co. Ltd.
Finglas Dublin II
*TRAXAM is a Registered Trademark
Issued/Last Revised:
December 1991 Edition No. 1
(This leaflet is produced in accordance with guidance issued by the Association of the British Pharmaceutical Industry.)