Gonal-F® 75 or 150
Qualitative and quantitative composition
Active ingredient: 75 IU or 150 IU follitropin alpha.
Excipients: Sucrose, Sodium dihydrogen phosphate, Disodium phosphate, phosphoric acid, sodium hydroxide.
Pharmaceutical form
Lyophilised sterile powder, for injection after reconstitution with accompanying diluent(Sterile Water for injection).
Uses
FSH, is a natural hormone, secreted by the anterior pituitary, a gland at the base of the brain. Gonal-F is used to treat infertility in women.
Authorisation holder and manufacturer
Authorisation holder: Ares-Serono (Europe) Ltd., 112 Harley Street, London W1N 1AF.
Manufacturer: Serono Pharma S.p.A., 70123 Bari, Italy.
Therapeutic indications
The medication should only be used under the strict supervision of a physician.
Gonal-F is used to bring about the development of several follicles (and therefore several eggs) for women undergoing assisted reproductive technologies (ART) such as in vitro fertilization (IVF), gamete intra-fallopian transfer (GIFT) or zygote intra-fallopian transfer (ZIFT).
Contraindications
Gonal-F should not be used when one of the following conditions exist:
- pregnancy
- ovarian enlargement or cyst not due to polycystic ovarian disease
- gynaecological bleeding of unknown cause
- ovarian, uterine or mammary cancer
- tumours of the hypothalamus and pituitary gland
- case of prior allergic reaction to medicines containing FSH.
The medicine should not be used when a condition exists which would make a normal pregnancy impossible, such as premature menopause, malformation of sexual organs or specific tumours of the womb.
Special warnings and precautions for use
Before the treatment is started, you and your partners fertility will be evaluated.
Patients using this treatment for development of several follicles are at an increased risk of developing ovarian hyperstimulation syndrome (OHSS) (see undesirable effects). Gonal-F treatment seldom gives rise to significant OHSS unless the medicine used to induce final follicular maturation (containing human chorionic gonadotropin-hCG) is administered. It is therefore prudent to withhold administration of hCG in cases when OHSS is developing and not to have sexual intercourse for at least four days.
The risk of multiple pregnancy following assisted reproductive technologies is related to the number of oocytes/embryos replaced.
Pregnancy loss (miscarriage) is higher than normal, but comparable with the rates found in women with fertility problems.
There have been no reports of allergic reactions to Gonal-F. If you had this type of reaction to similar medicines, inform your doctor.
Interaction with other medications and other forms of interaction
Concomitant use of Gonal-F with other agents used to stimulate ovulation may potentiate the ovarian response, whereas concurrent use of a gonadotrophin releasing hormone (GnRH) agonist-induced pituitary desensitisation may increase the dosage of Gonal-F needed to elicit an adequate ovarian response.
No drug incompatibilities have been reported for Gonal-F.
Gonal-F should not be administered as a mixture with other drugs in the same injection.
Use during pregnancy and lactation
Gonal-F should not be administered in case of pregnancy and lactation.
Dosage
A commonly used regimen for superovulation involves the administration of 150 225 IU of Gonal-F daily, commencing on days 2 or 3 of the cycle. Treatment is continued until adequate follicular development has been achieved (as assessed by blood monitoring and/or ultrasound examination), with the dose adjusted according to the patient’s response, to usually not higher than 450 IU daily. In general adequate follicular development is achieved on average by the tenth day of treatment (range 5 to 20 days).
A single injection of the medicine used to induce final follicular maturation and containing up to 10 000 IU human chorionic gonadotropin (hCG) is administered 24 48 hours after the last Gonal-F injection.
In other cases, down-regulation with a gonadotrophin-releasing hormone {GnRH) agonist is used. In these cases, Gonal-F is started approximately 2 weeks after the start of agonist treatment, both being continued until adequate follicular development is achieved. For example, following two weeks of treatment with an agonist, 225 IU Gonal-F are administered for the first 7 days. The dose is then adjusted according to the ovarian response.
Method and route of administration
Gonal-F is intended for subcutaneous administration and is for single use only.
If you administer Gonal-F to yourself, please care fully read the following instructions:
- Wash your hands. It is important that your hands and the items you use be as clean as possible.
- Assemble everything you need: find a clean area and lay out everything (two alcohol swabs, one diluent ampoule, one ampoule containing the medication, one syringe, one needle for reconstitution and a fine bore needle for subcutaneous injection).
- Open the ampoule: You should have one ampoule containing clear liquid (the diluent)and a set number of ampoules containing Gonal-F (the white powder). On the head of the diluent ampoule, you will see a small coloured dot. Directly below it is where the neck of the ampoule has been treated to make it easier to break. Gently flick the top section of the ampoule so that any fluid in the neck of the ampoule drops into the bottom chamber. Now press the ampoule firmly over the neck, and break the ampoule away from the coloured dot. Carefully place the open ampoule upright on the work surface
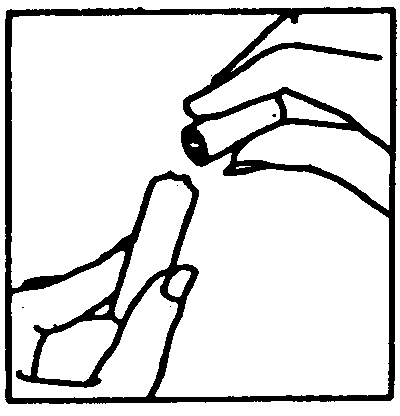
- Draw up the diluent: Attach the needle for reconstitution to the syringe, with the syringe in one hand pick up the open ampoule, insert the needle and draw up all of the diluent. Carefully set the syringe down on the work surface, taking care not to touch the needle.
continued