Calciparine®
Heparin Calcium Ph Eur
Presentation: Disposable syringes containing Heparin Calcium Ph Eur as follows: 5,000 iu heparin in 02 ml, 12,500 iu heparin in 0.5 ml, 20,000 iu heparin in 0.8 ml.
Uses: Prophylaxis and treatment of thromboembolic phenomena.
Administration: By subcutaneous injection only.
Dosage
Prophylaxis: Surgery 5,000 iu 2 hours before operation followed by 5,000 iu every a hours for 7 days or until the patient is ambulant. Medical conditions, especially myocardial infarction, 5,000 iu twice daily for 1 0 days or until the patient is ambulant. Anterior myocardial infarction with a risk of mural thrombosis of the left ventricle, 12,500 iu twice daily for at least 10 days. For the higher dosage regimen regular monitoring should be considered.
Treatment: The initial treatment dose is 2,500 iu per 10 kg body weight 12 hourly. Subsequent doses are adjusted to maintain a coagulation time approximately twice that of a control.
Special Cases: A higher dose may be necessary during pregnancy and in patients of abnormally high bodyweight, those suffering from cancer, diabetes mellitus or other diseases associated with marked hypercoagulability.
A lower dose may be necessary in the elderly, and in children and patients with low serum albumin or impaired renal or hepatic function.
Individual adjustment and control of dosage is required in these patients.
Contra-Indications: Hypersensitivity to heparin, advanced renal or hepatic dysfunction, severe hypertension, shock, bleeding diatheses (other than disseminated intravascular coagulation) and other conditions in which there is an increased danger d haemorrhage eg gastric and duodenal ulcer, sub-acute bacterial endocarditis, threatened abortion and major surgery involving the brain, spinal cord and eye.
Warnings: Care should be taken in elderly patients, pregnant women and patients receiving drugs which interfere with platelet aggregation or coagulation. Platelet counts should be measured in patients under heparin therapy for longer than five days and therapy stopped in those who develop significant thrombocytopenia.
Side Effects: Hypersensitivity reactions, thrombocytopenia (usually reversible but may be severe and may include thrombotic episodes); rarely skin necrosis; haemorrhage; raised transaminases; local effects including pain and bruising; osteoporosis or alopecia during long term use.
Pharmaceutical Precautions: Store below 25°C but do not freeze. Do not refrigerate as cold injections can be painful. Other preparations should not be mixed with Calciparine.
Further information is available on request from 303/181
Sanofi Winthrop Limited
One Onslow Street - Guildford, Surrey GU1 4YS.
Telephone: Guildford (0483) 505515.
PL 11723/0011
Pottery Road, Dun Laoghaire, Co Dublin, Republic of Ireland.
PA Numbers
Pre-Filled Syringes of 5,000 iu - PA 77/115/1
Pre Filled Syringes of 12,500 iu - PA 77/115/2
Pre-Filled Syringes of 20,000 iu - PA 77/115/3
Manufactured by Laboratoire Choay, Notre-Dame-de-Bondeville, France.
continued
Dosage Guide
Prophylaxis for Thromboembolism
Calciparine: Subcutaneous Administration
Surgical patients over 40 years of age:
5,000 iu two hours before operation, followed by 5,000 iu every 8 hours for 7 days. In patients still confined to bed the dosage should be continued unto they are ambulant.
Medical patients:
Especially rnyocardial infarction, 5,000 iu twice daily for 10 days or until the patient is ambulant.
For anterior rnyocardial infarction with a risk of mural thrombosis of the left ventricle 12,500 iu twice daily for at least 10 days. For the higher dosage regimen regular monitoring should be considered.
Pregnancy:
5,000 iu every 8 hours is a suitable starting dose in the first 3 or 4 months of pregnancy but higher doses are needed as pregnancy progresses, 10,000 iu two or three times daily being usual in the last trimester. Individual control is essential and the aim should be to maintain plasma heparin levels between 0.1 and 0.4 iu/ml as assessed by the anti-Xa assay and a whole blood clotting time of 15-20 minutes.
Dosage must be reduced during labour and standard prophylactic dosage is suitable postpartum
Treatment of DVT
Calciparine: Subcutaneous administration
Initial treatment dose is 2,500 iu per 10 kg bodyweight 12 hourly. Subsequent doses should be adjusted to maintain a coagulation time approximately twice that of control.
Initial treatment doses for DVT
|
Bodyweight |
Dosage |
|
|
Kg |
ML |
IU |
|
40 |
0.40 |
10,000 |
|
45 |
0.45 |
11,250 |
|
50 |
0.50 |
12,500 |
|
55 |
0.55 |
13,750 |
|
60 |
0.60 |
15,000 |
|
65 |
0.65 |
16,250 |
|
70 |
0.70 |
17,500 |
|
75 |
0.75 |
18,750 |
|
80 |
0.80 |
20,000 |
|
85 |
0.85 |
21,250 |
|
90 |
0.90 |
22,500 |
|
95 |
0.95 |
23,750 |
|
100 |
1.00 |
25,000 |
continued
Removal of packaging
prior to injection
It is recommended that the following steps are followed:
To divide the syringes, carefully fold, several times, the twin pack so that the syringes are back to back, then slowly using an even pressure divide the two syringes starting from the plunger end of the pack.

To remove the syringe from its plastic packaging, gently tear the top backing paper completely from the plastic tray Starting from the plunger end), then allow the syringe to roll onto the palm of your other hand.
|
|
Note:
The rubber cap over the needle may appear to be asymmetrical on the syringe, however, this occurs during packaging and does not mean the needle is bent.
|
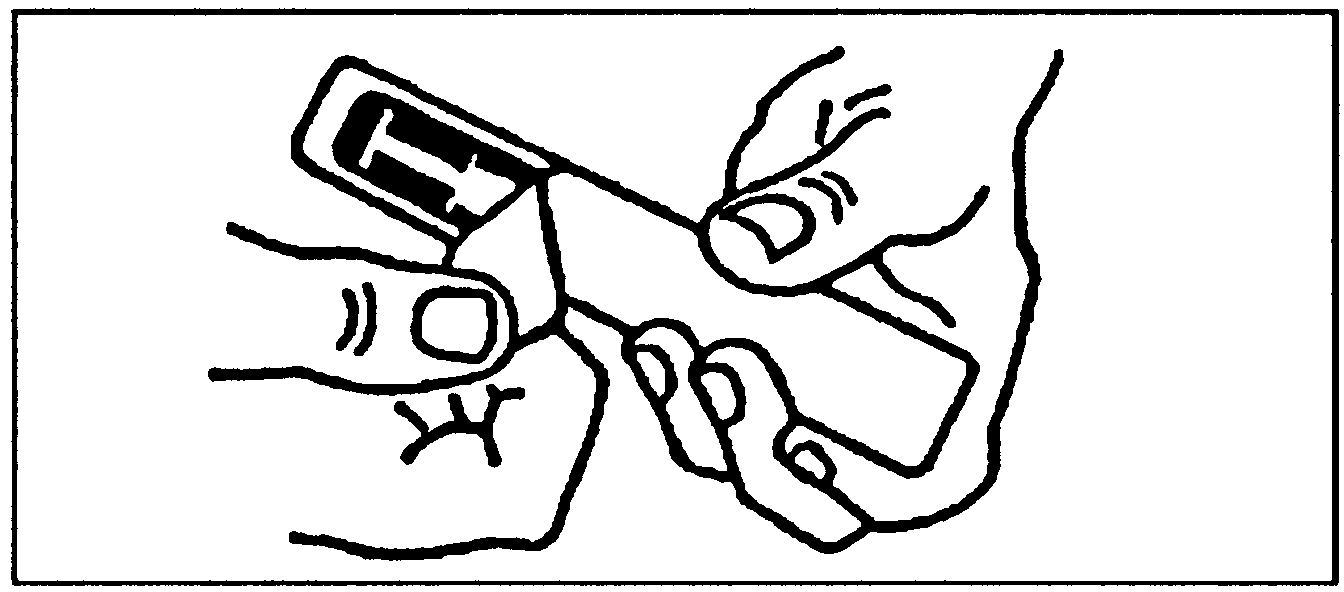
Removal of cap from syringe needle. |
|
Preparation of syringe
for subcutaneous injection
Hold the syringe vertically (grey cap uppermost).
|
|
Calciparine 5,000 iu/0.2ml - Pre-filled syringe:
This presentation is intended for administration d a unit dosage only. The entire contents of the syringe should be injected. There may be a small air bubble in the syringe but this does not have to be removed.
Calciparine 12,500 iu/0.5 ml and 20,000 iu/0.8 ml - Pre-filled graduated syringes:
These presentations may be used to administer unit or adjusted dosages.
Hold the syringe in the vertical position (needle uppermost) and ensure the air bubble is at the top of the syringe. With the air bubble at the top of the syringe advance the plunger to the volume/dosage required, expelling any excess.
Method for subcutaneous
administration
|
|
|
|
|
|
|
|
|
|
|
![]()