Reconstitution
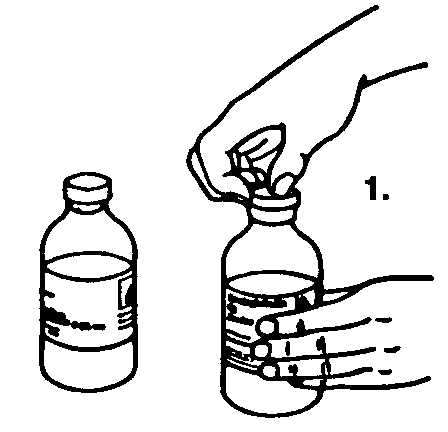
1. Tear off the protective caps from the sterile saline for injection and Sandoglobulin® bottles. Disinfect both rubber stoppers.
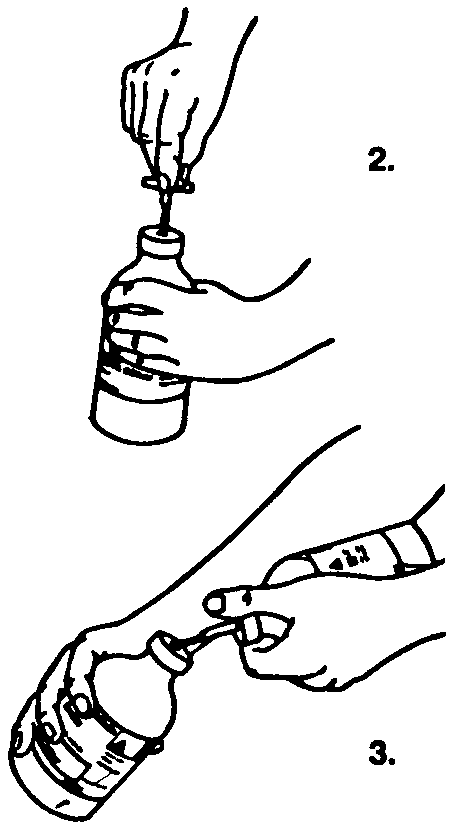
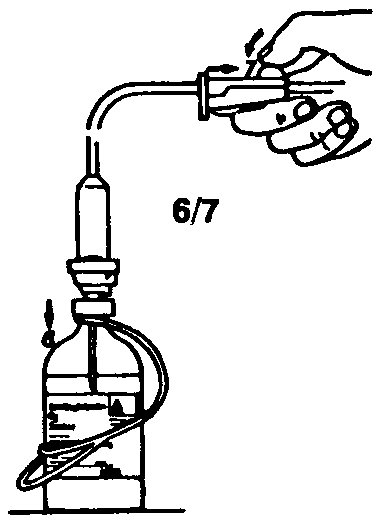
2. Remove the protective cover from one end of the transfer set and insert the spike through the rubber stopper of the bottle of saline.
3. Remove the protective cover from the other end of the transfer set. Lift both bottles, holding at a slight angle to the work surface, and insert spike into the Sandoglobulin® bottle.
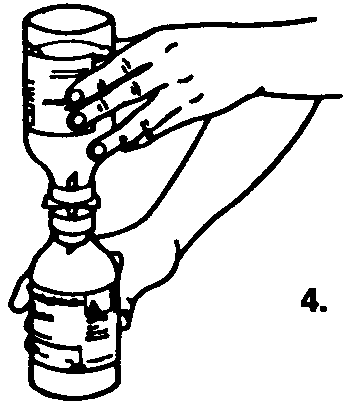
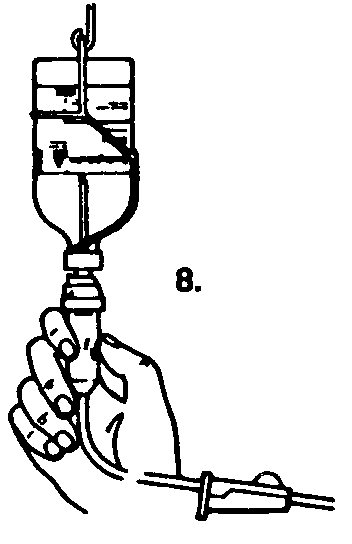
4. Invert the bottles so that the saline flows into the Sandoglobulin® bottle.
Turn both bottles vertically so that the Sandoglobulin® is on the bottom and the saline is on top.
Hold both bottles firmly to prevent loss of vacuum while the saline is drawn into the Sandoglobulin® bottle. In this manner the vacuum in the Sandoglobulin® bottle, together with gravity, works to accelerate the rate of saline transfer.
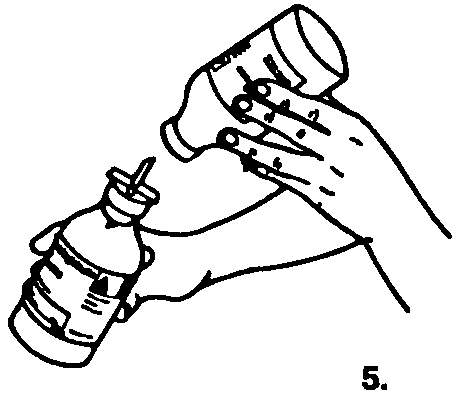
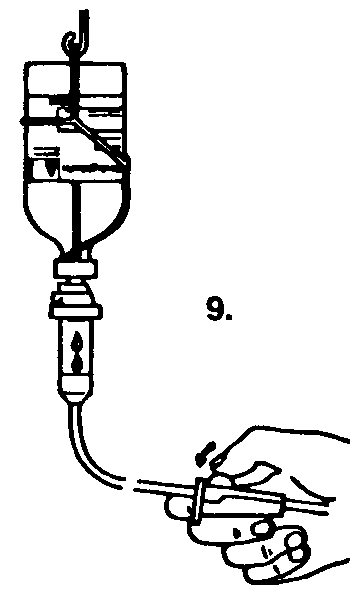
5. Once the saline is transferred, lift the saline bottle off the spike to release the vacuum. This will reduce foaming and facilitate dissolution.
Remove the spike.
The powder should dissolve within a few minutes to form an opalescent solution.
In exceptional cases, dissolution may take up to 20 minutes. It is important that all particles of Sandoglobulin® are completely dissolved and no clumps remain.
You can help this process by gently swirling the bottle but DO NOT SHAKE. Shaking can cause foaming and particle formation, both of which can impede the dissolving and administration process.
continued