|
Vagifem
Estradiol
What you should know about Vagifem
Please read this leaflet carefully before you start to use this product. The leaflet does not contain all of the information about the product that you may need to know, so please ask your doctor or pharmacist if you have any questions.
What is in your medicine
Each Vagifem vaginal tablet contains 25 microgrammes of estradiol.
Other ingredients are: E464, lactose, maize starch, magnesium stearate and polyethylene glycol 6000.
Vagifem is a vaginal tablet containing a small dose of estradiol (an oestrogen). The vaginal tablet is inserted into the vagina, and the estradiol acts locally on the vagina. Each white Vagifem tablet comes in an applicator which is used once only. There are 15 applicators with vaginal tablets in each box. Vagifem is one of a group of hormone replacement therapies called topical (or local) HRT.
The manufacturer and product licence holder is: Novo Nordisk A/S, Novo Allé, DK-2880 Bagsvaerd, Den mark
What is Vagifem for?
Vagifem is prescribed to relieve or eliminate symptoms in the vagina such as dryness or irritation. In medical terms this is known as atrophic vaginitis. It is caused by a loss of the female sex hormone, oestrogen, which occurs around the menopause.
The menopause is the time of a woman's last period. Before the menopause oestrogen is important in keeping the vagina moist. When the amount of this hormone falls before and after the menopause, some women get a drying and thinning of the vagina which can lead to soreness, discomfort during sexual intercourse, and possible infection. These symptoms may often start years after the menopause when amounts of oestrogen in the body have become very low. By treating your vagina with the oestrogen your body no longer produces, Vagifem relieves or eliminates these symptoms.
Important questions before using Vagifem
- Have you had cancer of the breast or endometrium (lining of the womb)
- Have you recently had any vaginal bleeding, other than during a period?
- Have you had phlebitis (inflamed veins), DVT (deep vein thrombosis), blood clots in the lungs, or stroke, associated with previous oestrogen use?
- Are you pregnant?
If you have answered Yes to any of these questions, tell your doctor or pharmacist before starting Vagifem.
Precautions
Your doctor will carry out gynaecological and breast examinations as appropriate for you, and will treat any vaginal infection before you start with Vagifem.
It has been established that the risk of endometrial cancer (cancer of the lining of the womb) is increased after treatment with higher doses of oestrogen given by mouth. Vagifem is a local treatment with a low dose of oestrogen and even though a minor degree of absorption of the oestrogen may occur, this side effect is most unlikely.
Tell your doctor if you have any persistent or recurring vaginal bleeding during use of Vagifem.
There has been concern about the possible risk of breast cancer with oestrogen treatment. Many studies have not shown any increase in breast cancer but some have shown a small increase after prolonged treatment (five years or more). All of these studies have involved higher doses of oestrogen given by mouth. Because of the low dose and local treatment with Vagifem any effect on the risk of breast cancer is unlikely.
Vagifem should not be used by children or males. |
Vagifem and other diseases
There are certain conditions with which it is probably quite safe to use Vagifem, but please do remind your doctor if you have any of the conditions mentioned below, as he may want to keep a closer eye on your condition than would otherwise be the case. If your condition worsens whilst using Vagifem, stop using Vagifem and tell your doctor. The conditions are: inflamed veins, blood clots in the legs or lungs, stroke or heart attack, or a past history of these conditions; liver disease or previous liver disease where liver function tests have not returned to normal; inherited blood disorders; epilepsy; migraine; diabetes; asthma; heart trouble; high blood pressure.
Contraception
If you have passed the menopause you cannot become pregnant. If you are fertile or are not sure if you have passed the menopause, you should be aware that Vagifem is not a contraceptive. If you become pregnant whilst using Vagifem you must stop using It.
Using your Vagifem
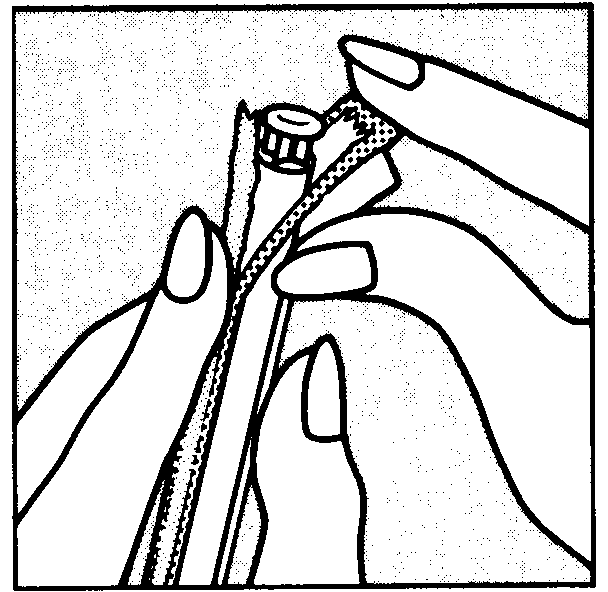
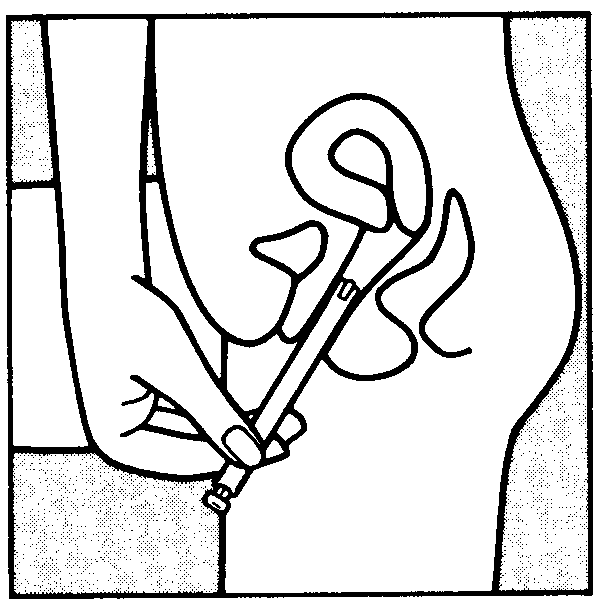
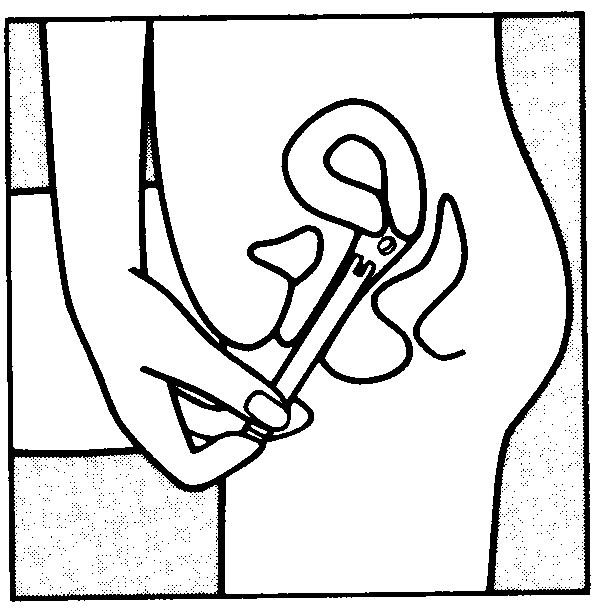
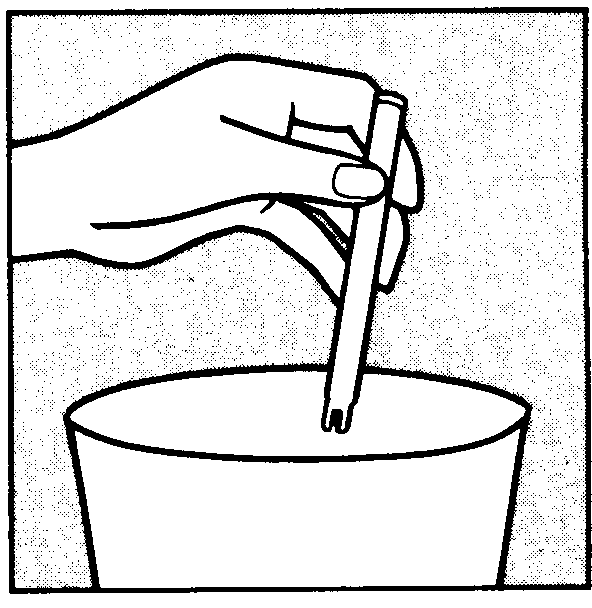
You can start using Vagifem on any convenient day. The white vaginal tablet is inserted deeply within the vagina using the disposable applicator.
The usual dose is one tablet inserted into the vagina each day for two weeks, and then one tablet inserted twice per week for a further 10 weeks. At the end of this treatment your doctor will usually decide whether further treatment is required or not.
To insert the tablet follow the diagrams shown below. Each applicator containing one tablet, is wrapped in a blister pack backed with foil. The applicator with tablet must be removed from this pack just before insertion.
Overdosage
Because of the low dose of estradiol in Vagifem, using too many should not cause serious problems. However, if you think you have used more than you should, contact your doctor for advice.
Missing a tablet
If you forget to insert a tablet on any given day, continue from the next day on which you were due to use Vagifem. You can stop treatment with Vagifem at any time' but symptoms may return. Tell your doctor if you stop or want to stop treatment.
Possible side effects
Only a few side effects have been reported during use of Vagifem. These include slight vaginal bleeding, vaginal discharge and skin rash.
Tell your doctor or pharmacist if you experience recurring vaginal bleeding, any unusual discomfort you do not understand or any other unwanted effect not mentioned above.
How to keep your tablets
Store Vagifem in a dry place, protected from light (keep the bubble strips in the box).
Store at room temperature, below 25 C. Do not keep in a refrigerator.
The expiry date is printed on the foil of the bubble pack and on the outer box. Do not use the tablets after this date.
Keep your Vagifem away from children.
If your doctor decides to stop the treatment return any left over tablets and applicators to the pharmacist.
Date of last revision of this leaflet: November 1995
continued |