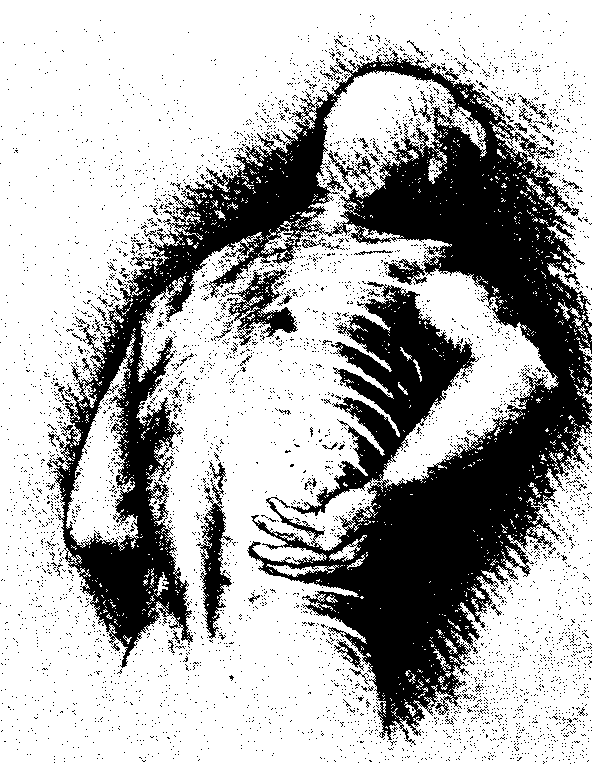
Axsain®
(Capsaicin 0.075%)
Topical Analgesic Cream
For External Use Only
![]()
EURODERMA LIMITED
ABOUT AXSAIN
Axsain cream contains capsaicin (0.075%), a naturally occurring substance found in plants of the Solanaceae family. Research has shown that Axsain may work by reducing levels of a chemical (substance P), which is involved in transmitting pain impulses to the brain. Axsain therefore has a topical analgesic effect.
USES OF AXSAIN
Axsain can be helpful in relieving neuralgia or the pain arising from nerves near to the surface of the skin. This pain often follows Herpes Zoster (shingles), after the sores have healed.
WARNINGS
AXSAIN IS FOR EXTERNAL USE ONLY AND SHOULD NOT COME INTO CONTACT WITH THE EYES OR BROKEN OR DAMAGED SKIN
.If it does, wash it off with plenty of soap and water. Do not put tight bandages over Axsain cream. If your condition worsens or symptoms persist for more than 14 days, or clear-up and then recur within a few days, stop using Axsain and consult your doctor.
Keep this and all drugs away from children.
Preservative: Benzyl alcohol 1% w/w.
For expiry date see end of carton or tube.
HOW TO USE AXSAIN
Apply the cream to the affected area 3 to 4 times daily. A warm or burning sensation may be felt for a time after application. This sensation tends to occur more often when the cream is applied less than 3 to 4 times daily. After applying Axsain with your fingers, wash your hands thoroughly.
Axsain is not recommended for use in children.
DIRECTIONS FOR USE
Using fingers rub in Axsain to the affected skin areas.
Warning: Do not use on broken or damaged skin and do not bandage tightly.
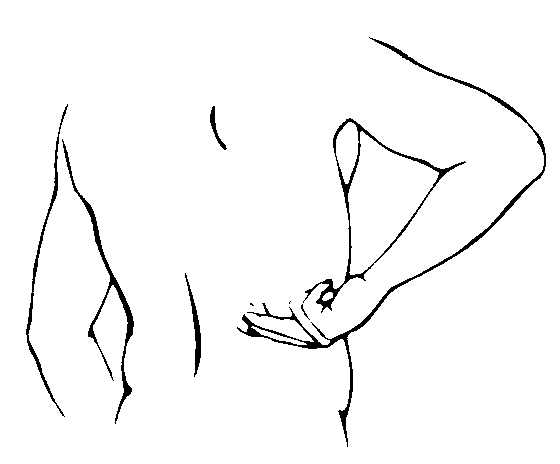

4. Maximum response is usually achieved within 14 to 28 days and application 3 to 4 times daily is advised to sustain pain-relief. We suggest that you use the diary overleaf to help you record and check your applications.
PL No: 10670/0003
Manufactured for Euroderma Ltd. by
Dermatological Products of Texas, Inc.
San Antonio, Texas 78215